When you hear biosimilars, highly similar versions of complex biologic drugs that are not exact copies but proven to work the same way in the body. Also known as biologic generics, they’re not like the simple generic pills you pick up at the pharmacy. These are made from living cells, not chemicals, and they treat serious conditions like cancer, rheumatoid arthritis, and diabetes. Unlike regular generics, which are exact copies of small-molecule drugs, biosimilars are built to match the structure and function of their reference biologics—drugs like Humira, Enbrel, or Remicade—that can cost tens of thousands a year.
The biologics, medications made from living organisms, often proteins or antibodies, used to treat chronic and life-threatening diseases they copy are too complex to replicate perfectly. That’s why regulators like the FDA don’t call them generics—they call them biosimilars, medications that are highly similar to an approved biologic with no clinically meaningful differences in safety or effectiveness. To get approval, manufacturers must prove their version works just as well in clinical trials, with no extra side effects. It’s not about cutting corners—it’s about matching the original with precision.
Why does this matter? Because drug affordability, the ability of patients to access and pay for necessary medications without financial hardship is broken for many. Biologics are expensive not because they’re better, but because patents lock out competition for years. Biosimilars break that lock. In the U.S., they’ve already saved billions. In Europe, where they’ve been around longer, they’ve cut prices by 30% to 80%. That means more people get treatment, not just those with top-tier insurance.
But it’s not all smooth sailing. Some doctors still hesitate to switch patients from the original biologic, even when guidelines say it’s safe. Others worry about subtle differences in how the body reacts—especially for drugs with a narrow therapeutic window. And while biosimilars are cheaper, they’re still pricey compared to pills. Still, the trend is clear: more biosimilars are coming. The FDA has approved dozens, and hundreds more are in the pipeline. If you’re on a biologic right now, you might soon have a lower-cost option.
What you’ll find below are real stories and facts about how biosimilars fit into the bigger picture of medication safety, access, and cost. You’ll read about how patent battles delay their arrival, how some patients react differently to them, and why even small changes in inactive ingredients can matter. You’ll also see how they connect to broader issues like generic drug approval, drug shortages, and patient assistance programs. This isn’t theoretical—it’s happening in clinics, pharmacies, and living rooms right now. Whether you’re a patient, caregiver, or just trying to understand your bill, these posts give you the facts you need to ask the right questions.

Understand how 2025 Medicare Part D formulary updates are forcing generic and biosimilar switches, what drugs are affected, and how to protect your coverage before January 1.
Biosimilars are the closest thing to generics for complex biologic drugs. They're highly similar, FDA-approved, and can save patients up to 60% on costs. Learn how they work, why they're not exact copies, and how to use them safely.
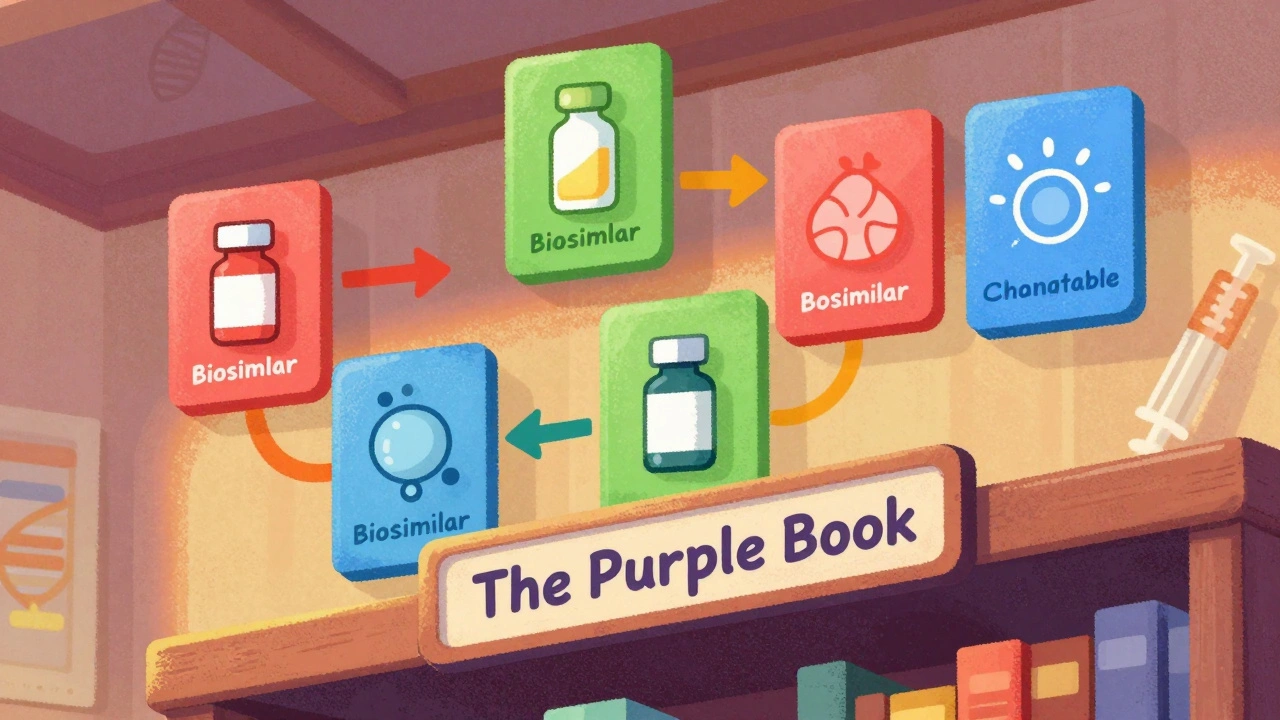
The FDA's Purple Book is the official guide to biosimilars and interchangeable biological drugs. Learn how it works, what the difference is between biosimilars and interchangeable products, and how pharmacists use it to make safe substitutions.

A detailed side‑by‑side comparison of Neoral (Cyclosporine) with Tacrolimus, Sirolimus, Mycophenolate, and Azathioprine, covering mechanisms, dosing, side effects, cost and monitoring.
Loperamide, found in OTC antidiarrheals like Imodium, is being misused by people trying to self-treat opioid withdrawal. At high doses, it causes life-threatening heart rhythms and can be fatal. Learn the warning signs and why this isn't just another drug trend.
Acetaminophen combination products like Vicodin and Percocet can cause silent liver damage when taken with other meds. Learn how to avoid overdose, recognize risks, and use safer pain management strategies.

Learn how to report adverse drug reactions to the FDA's MedWatch program. Understand what counts as reportable, how to fill out the form, and why your report matters for drug safety.

Learn how to ethically obtain free medication samples from trusted platforms and track expiration dates to avoid health risks. Save money on prescriptions without compromising safety or integrity.