When a drug company gets FDA exclusivity, a period of market protection granted by the U.S. Food and Drug Administration that blocks generic versions from entering the market, even if the patent has expired. It's not a patent—it's a separate rule that lets companies charge high prices without competition for a set number of years. This is why you might see a brand-name drug still on the shelf long after its patent ends. The FDA doesn't control prices directly, but exclusivity is one of the biggest reasons why some medications stay expensive.
FDA exclusivity isn't the same for every drug. It varies based on the type of medicine. For example, new chemical entities get five years of exclusivity, while orphan drugs for rare diseases can get seven. If a company does new studies for kids, they might get an extra six months. These rules are built into the law to encourage innovation, but they also delay cheaper alternatives. You see this in posts about generic drugs, medications that contain the same active ingredient as brand-name drugs but cost up to 85% less. Also known as generic medication, they're the main way people save money on prescriptions—but they can't launch until exclusivity runs out. That’s why some people struggle to afford their meds, even when the drug has been around for decades.
Exclusivity also affects how quickly alternatives appear. If a drug has exclusivity, compounding pharmacies or alternative treatments might be your only options, as seen in posts about drug shortages, situations where manufacturers can’t keep up with demand, often due to production issues or exclusivity delays. And when exclusivity ends, the market shifts fast—generic versions flood in, prices drop, and insurance plans update their formularies. That’s why switching health plans or checking your drug coverage matters so much. The timing of exclusivity expiration can save you hundreds a year.
It’s not just about patents or money. Exclusivity shapes how doctors prescribe, how patients access care, and even how research gets done. If a company knows it has exclusivity, it’s less likely to invest in cheaper formulations or better delivery methods. Meanwhile, patients with narrow therapeutic index drugs—like levothyroxine or warfarin—may notice small differences between brand and generic, even after exclusivity ends. That’s why some people stick with the brand, even when it costs more.
What you’ll find below are real stories about how exclusivity plays out in everyday healthcare: why some drugs disappear from shelves, how manufacturers use exclusivity to extend profits, and what you can do when your medication suddenly becomes unaffordable. These aren’t abstract policies—they’re decisions that affect your wallet, your health, and your access to treatment.

The 180-day exclusivity rule under the Hatch-Waxman Act was meant to speed up generic drug entry-but now it often delays it. Learn how patent challenges, FDA rules, and corporate strategy keep prices high and patients waiting.
Ceramides restore the skin barrier in eczema by replacing missing lipids. Proper bathing-short, lukewarm soaks followed by immediate moisturizing-boosts results. Learn how to use ceramides effectively for lasting relief.

Buyers like Medicare use the presence of generic drugs to negotiate lower prices for brand-name medications. Generic competition drives prices down by over 90% in many cases, making it a key tool for controlling drug costs.

Explore how self‑driving cars could reshape traffic jams, the tech behind them, policy levers, real‑world pilots, and what it means for commuters and cities.

Learn how to manage salt intake while taking ramipril to lower blood pressure, reduce side effects, and protect your heart and kidneys. Practical diet tips, hidden sodium sources, and what to avoid.
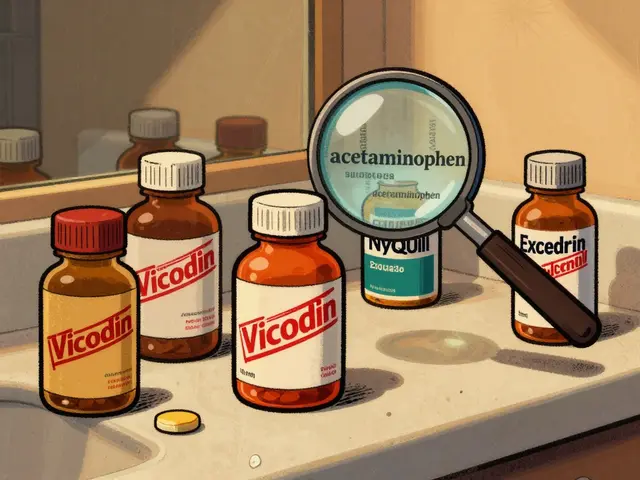
Acetaminophen combination products like Vicodin and Percocet can cause silent liver damage when taken with other meds. Learn how to avoid overdose, recognize risks, and use safer pain management strategies.