When a generic drug company wants to sell a cheaper version of a brand-name medicine before the patent expires, they file a Paragraph IV certification, a legal notice filed with the FDA asserting that a brand-name drug’s patent is invalid, unenforceable, or won’t be infringed by the generic version. Also known as a Paragraph IV notice, this is the main tool generic manufacturers use to break patent monopolies and speed up access to low-cost medications. It’s not a request for approval—it’s a challenge. And when done right, it can drop drug prices by 80% or more within months.
This process ties directly to the ANDA, Abbreviated New Drug Application, the streamlined FDA pathway for generic drugs that doesn’t require repeating expensive clinical trials. While brand-name companies spend billions on trials to prove safety and effectiveness, generics only need to show they work the same way in the body. The Paragraph IV certification is the trigger that lets them skip the waiting game. Once filed, the brand-name maker has 45 days to sue for patent infringement. If they do, the FDA can delay generic approval for up to 30 months—but if they don’t, the generic hits the market immediately. That’s why so many generic companies file these certifications: it’s the fastest way to get a foot in the door.
It’s not just about money. Paragraph IV certifications affect real people. If you’ve ever switched from a name-brand pill to a generic and noticed a difference in how it works—or didn’t—it might be tied to this process. Some generics use slightly different inactive ingredients, which can matter for people with allergies or those on narrow therapeutic index drugs like warfarin or levothyroxine. That’s why the FDA requires bioequivalence testing, and why the certification process includes strict standards for manufacturing and absorption. The system isn’t perfect, but it’s designed to balance innovation with access.
Behind every cheap generic you pick up at the pharmacy is likely a Paragraph IV filing. It’s how drugs like metronidazole, omeprazole, and tadalafil became affordable. It’s how patients on statins, antidepressants, or blood pressure meds save hundreds a year. And it’s why drug manufacturers fight so hard to protect their patents—because once one generic wins, others follow fast. The posts below dig into how this system shapes your prescriptions, why some generics cause unexpected reactions, how drug shortages happen when patents are challenged, and what happens when companies delay generics through legal tricks. You’ll see real examples from people who’ve benefited—and those who’ve been caught in the middle.

The 180-day exclusivity rule under the Hatch-Waxman Act was meant to speed up generic drug entry-but now it often delays it. Learn how patent challenges, FDA rules, and corporate strategy keep prices high and patients waiting.

Learn how progesterone affects gut motility, bile flow, and microbiome health, recognize deficiency signs, and discover natural and medical ways to improve digestion.

Learn how warfarin interacts with kidney disease, dosing tips, risks, and alternatives to keep you safe and in control of your anticoagulation.

TZDs like pioglitazone help control blood sugar but often cause weight gain and swelling. Learn proven strategies to reduce fluid retention, lower doses safely, and combine with better alternatives like SGLT2 inhibitors.
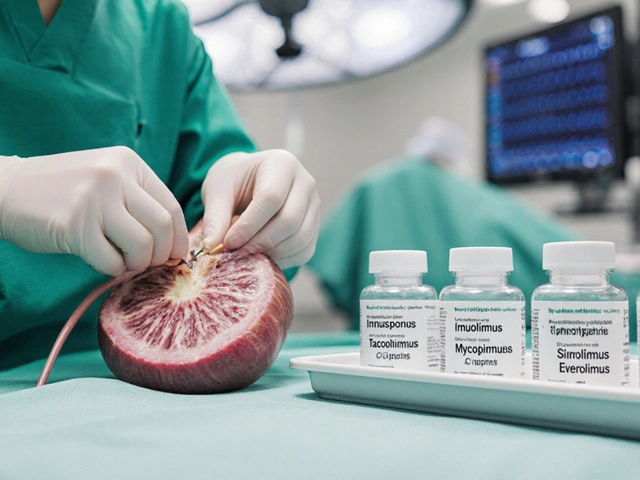
A side‑by‑side look at Imusporin (cyclosporine) versus tacrolimus, mycophenolate, sirolimus, everolimus and others, covering mechanisms, side effects, UK costs and when to switch.

A detailed look at Chloramphenicol, its clinical uses, safety concerns, and how it stacks up against common alternatives such as Azithromycin, Ciprofloxacin, and Doxycycline.