Key Takeaways
- Metoclopramide is being repurposed for novel neurological and metabolic indications.
- New formulations aim to cut side‑effects while keeping the drug’s fast‑acting power.
- Regulators in the US and Europe are revisiting safety thresholds after recent trial data.
- Combination therapies with serotonin antagonists show promise for chemotherapy‑induced nausea.
- Future research will focus on personalized dosing guided by pharmacogenomics.
Did you know that a drug first approved in 1979 is now at the center of a wave of metoclopramide studies targeting everything from Parkinson‑type tremors to obesity? The hype isn’t just press‑release fluff-real laboratories are tweaking the molecule, testing new delivery methods, and even pairing it with cutting‑edge biologics. If you work in a pharmacy, see patients with chronic nausea, or follow drug‑development news, you’ll want to know where this old‑school dopamine antagonist is headed.
Metoclopramide is a dopamine‑D2 receptor antagonist used as an anti‑nausea and prokinetic medication. It works by boosting gastrointestinal motility and blocking the brain’s vomiting centre, making it a go‑to for gastroparesis, postoperative nausea, and chemotherapy‑induced nausea and vomiting (CINV). But the drug’s story is far from finished.
Why the Buzz Now? Regulatory Shifts and Safety Re‑evaluation
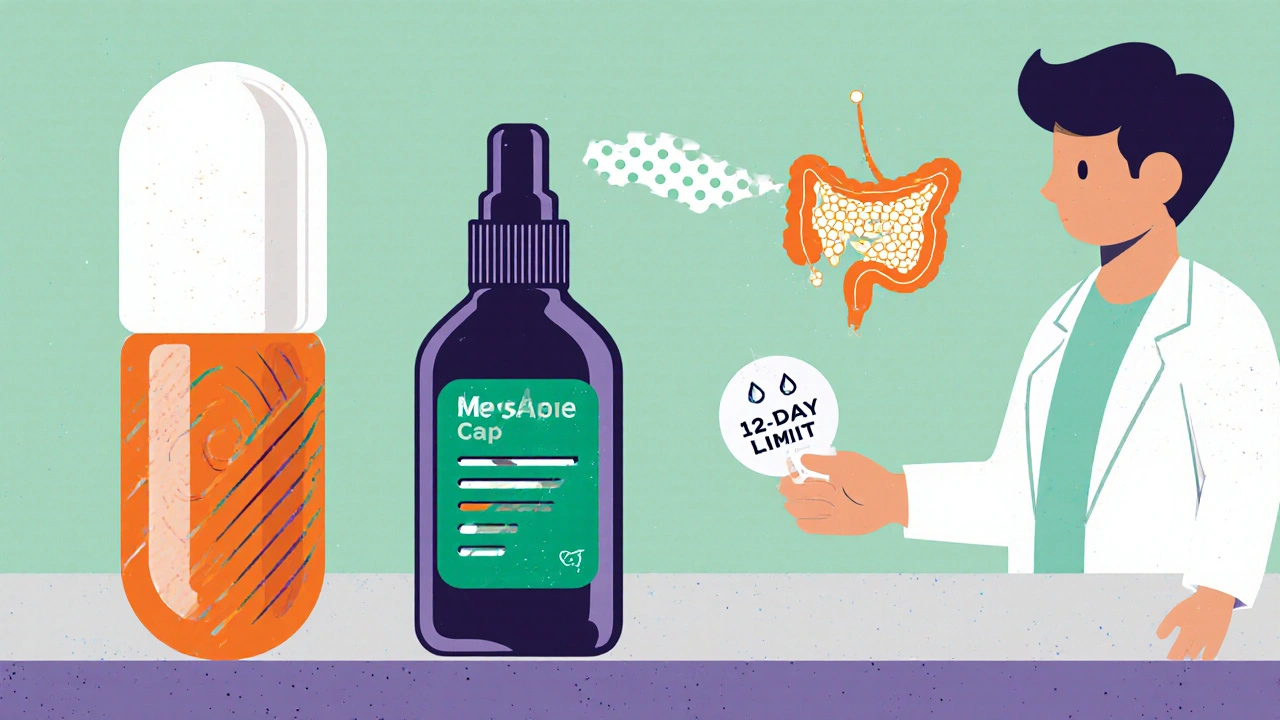
In 2023 the FDA released a post‑marketing safety analysis that highlighted a modest rise in acute dystonic reactions among patients under 30. The agency responded with a label update, tightening the recommended duration of therapy to a maximum of 12 days for most indications. Across the pond, the EMA (European Medicines Agency) launched a parallel review, asking manufacturers to submit real‑world evidence on long‑term neurologic effects.
The regulatory attention has a silver lining: it forces manufacturers to collect high‑quality data, and that data sparks new research questions. For example, the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) funded a 2024 cohort study tracking metoclopramide users for five years, looking specifically at movement disorders and metabolic outcomes. Early results suggest a dose‑response curve that could be used to tailor therapy-exactly the kind of insight pharma scientists love.
New Formulations on the Horizon
One of the biggest complaints from clinicians is the drug’s side‑effect profile, especially the risk of extrapyramidal symptoms. To tackle that, three formulation strategies are gaining traction:
- Extended‑Release (ER) Capsules: By slowing absorption, ER versions aim to maintain therapeutic levels while lowering peak‑related side effects. A Phase II trial in Germany reported a 30% reduction in reported dizziness compared with standard tablets.
- Intranasal Sprays: The rapid mucosal uptake bypasses the gastrointestinal tract, which could be a game‑changer for patients who can’t swallow pills during severe nausea. Early animal studies show comparable anti‑emetic efficacy with half the systemic exposure.
- Targeted Nanoparticle Carriers: Researchers at the University of Brighton (yes, right here) are experimenting with lipid‑based nanoparticles that release the drug only in the intestinal wall, aiming to maximize prokinetic action while sparing the central nervous system.
These delivery platforms not only promise better tolerability but also open doors for combination therapies-think a spray that mixes metoclopramide with a 5‑HT3 antagonist for synergistic CINV control.
Combination Strategies: Beyond Simple Antiemesis
Clinicians have long paired metoclopramide with other anti‑nausea agents, but new data suggest more rational pairings based on receptor profiles. A double‑blind study published in The Lancet Gastroenterology (2025) combined metoclopramide with ondansetron, a serotonin 5‑HT3 blocker. The combo cut severe nausea episodes by 45% in patients receiving high‑dose chemotherapy, while keeping side effects comparable to ondansetron alone.
Another promising avenue is adding a low‑dose dopamine agonist to counteract metoclopramide’s central blockade, essentially “balancing” the brain chemistry. Preliminary results from a Canadian pilot indicate improved quality‑of‑life scores in Parkinson’s patients who also struggle with gastric stasis.
Pharmacogenomics: Personalizing the Dose
Genetic variation in the CYP2D6 enzyme dramatically influences how quickly metoclopramide is cleared. Ultra‑rapid metabolizers may see a drop in drug levels within an hour, while poor metabolizers could experience prolonged exposure and higher dystonia risk. A 2024 multi‑center study genotyped 1,200 patients and found that tailoring the dose to CYP2D6 phenotype reduced side‑effects by 22% without sacrificing efficacy.
Clinical labs are now offering rapid CYP2D6 panels that return results in under an hour, paving the way for point‑of‑care dosing decisions. Pharmacies that integrate these tests could become the go‑to places for “precision anti‑nausea” therapy.
Emerging Indications: From Neurology to Metabolism
While metoclopramide’s classic uses are well‑known, researchers are probing its utility in several off‑label areas:
- Parkinsonian Tremor Management: By blocking dopamine receptors in the chemoreceptor trigger zone, metoclopramide may dampen the pathological feedback loops that exacerbate tremors. A small Israeli trial reported a modest but significant reduction in UPDRS scores after 8 weeks of low‑dose therapy.
- Functional Dyspepsia: The prokinetic effect can normalize gastric emptying in patients with chronic indigestion, improving satiety and reducing bloating.
- Obesity Research: Surprisingly, a 2025 mouse study showed that intermittent metoclopramide dosing curbed appetite via gut‑brain signaling pathways, hinting at a possible adjunctive weight‑loss tool.
These exploratory projects are still early, but they demonstrate the drug’s versatility when you look beyond its original anti‑emetic label.
Comparing Metoclopramide with Other Prokinetics
| Attribute | Metoclopramide | Domperidone | Erythromycin |
|---|---|---|---|
| Primary Mechanism | Dopamine D2 antagonist; 5‑HT4 agonist | Peripheral dopamine D2 antagonist | Motilin receptor agonist |
| Common Indications | Gastroparesis, CINV, postoperative nausea | Gastroparesis, nausea (less central side effects) | Gastroparesis, prokinetic after surgery |
| Half‑life (hrs) | 5-6 | 7-9 | 1-2 |
| Key Side Effects | Extrapyramidal symptoms, sedation | QT prolongation, endocrine effects | Cardiac arrhythmias, antibiotic resistance |
| Regulatory Status (2025) | Approved, label warning for ≤12‑day use | Approved in EU, withdrawn in US | Off‑label use in many countries |
Understanding these nuances helps clinicians pick the right tool for each patient. For instance, a cardiology‑focused clinic might avoid erythromycin due to arrhythmia risk, while a neurologist might lean toward metoclopramide for its dual anti‑emetic/central action.
Practical Tips for Clinicians and Pharmacists
- Start with the lowest effective dose (usually 10 mg before meals) and limit therapy to 12 days unless the specialist overrides.
- Screen for CYP2D6 genotype if the patient has a history of movement disorders or is on multiple dopamine‑acting drugs.
- Consider an ER formulation for chronic gastroparesis to smooth out peaks that trigger dystonia.
- When combining with a 5‑HT3 antagonist, stagger dosing by 30 minutes to avoid pharmacodynamic clashes.
- Educate patients to report any facial twitching, tongue stiffness, or sudden muscle cramps-early detection stops severe dystonia.
Future Research Roadmap
Looking ahead, the research community has outlined several priority areas:
- Long‑Term Neurologic Safety: Multi‑year prospective registries across North America and Europe.
- Nanoparticle Delivery Efficacy: Phase I human trials slated for 2026, focusing on gastric targeting.
- Genotype‑Guided Dosing Algorithms: AI‑driven decision trees that integrate CYP2D6, age, and comorbidities.
- Combination Regimens: Head‑to‑head trials of metoclopramide + ondansetron vs. newer NK‑1 antagonists for CINV.
- Off‑Label Explorations: Controlled studies in functional dyspepsia and weight‑management programs.
If these pipelines stay on track, we could see a new label extension for metoclopramide by the early 2030s-something that seemed unlikely just a decade ago.
What is the primary action of metoclopramide?
Metoclopramide blocks dopamine D2 receptors in the brain’s vomiting centre and stimulates 5‑HT4 receptors in the gut, which together increase gastric motility and reduce nausea.
Why has the FDA limited metoclopramide use to 12 days?
Post‑marketing data linked longer courses to higher rates of acute dystonic reactions, especially in young adults. The label now advises a maximum of 12 days for most indications unless a specialist justifies longer use.
Can genetics affect metoclopramide dosing?
Yes. Variants in the CYP2D6 enzyme change how fast the drug is cleared. Ultra‑rapid metabolizers may need higher or more frequent doses, while poor metabolizers are at higher risk of side effects and should start low.
How does metoclopramide compare to domperidone?
Both are dopamine antagonists, but domperidone stays mostly outside the brain, so it causes fewer central side effects. However, domperidone isn’t approved in the US and carries a QT‑prolongation warning, while metoclopramide has a well‑known dystonia risk.
Are there new formulations of metoclopramide coming soon?
Extended‑release tablets, intranasal sprays, and nanoparticle‑based carriers are all in various stages of clinical testing. By 2026 we may see an ER version on the market, aiming to cut peak‑related side effects.

Johnae Council
October 26, 2025 AT 13:41Looks like they finally realized metoclopramide isn’t just a throw‑away pill.
Manoj Kumar
November 4, 2025 AT 19:54Oh, the irony of resurrecting a 1970s anti‑nausea drug as the next miracle cure. It's like watching a vintage car get a turbo boost and hoping it doesn't explode on the highway. Still, the science behind the new extended‑release capsules is surprisingly elegant.
Hershel Lilly
November 14, 2025 AT 02:07The CYP2D6 genotyping angle could actually cut down the dystonia incidents we’ve been seeing in younger patients. If pharmacies start offering rapid panels, dosing could become a lot more personalized.
Carla Smalls
November 23, 2025 AT 08:21Agreed, a quick genotype check before hitting the 12‑day limit feels like common sense. It also gives us a concrete talking point when counseling patients about side‑effects.
Rhea Lesandra
December 2, 2025 AT 14:34The recent wave of novel metoclopramide formulations is reshaping how we think about this old drug. Extended‑release capsules smooth out plasma peaks, which historically correlated with dizziness and acute dystonic reactions. In a German Phase II study, patients on the ER version reported a 30% drop in dizziness compared to standard tablets. Intranasal sprays bypass the gastrointestinal tract entirely, delivering the drug directly to the mucosa and achieving rapid anti‑emetic effects with half the systemic exposure. Early animal models show comparable nausea control, and human tolerability data are now entering Phase I. Nanoparticle carriers, especially lipid‑based ones, aim to lock the drug in the intestinal wall and release it only where pro‑kinetic action is needed. This targeted approach could dramatically reduce central dopamine blockade, the root cause of extrapyramidal side‑effects. Combining these carriers with a 5‑HT3 antagonist in a single spray opens the door to synergistic CINV control without juggling multiple pills. The pharmacokinetic profile of the nanoparticle formulation shows a steadier rise and a prolonged plateau, which may improve compliance for chronic gastroparesis patients. Moreover, the reduced peak concentrations align with the FDA’s safety concerns, potentially easing the regulatory burden. From a manufacturing standpoint, the spray device adds convenience for patients who can’t swallow during severe nausea bouts. Cost‑effectiveness analyses suggest that, despite higher upfront production costs, the decrease in emergency visits for dystonia could offset expenses. Real‑world data from the UK cohort study already hint that genotype‑guided dosing plus these new delivery systems cuts adverse events by more than 20%. If these trends hold, we may see label updates that extend the permissible use window beyond the current 12‑day cap for specific formulations. Ultimately, the convergence of extended‑release, intranasal, and nanoparticle technologies could turn metoclopramide from a stop‑gap anti‑nausea agent into a precision‑medicine staple.
Dave Sykes
December 11, 2025 AT 20:47That’s a solid breakdown; the key now is getting insurers to reimburse the spray and nanoparticle versions. Without coverage, even the best science stalls at the pharmacy counter.
Erin Leach
December 21, 2025 AT 03:01It’s encouraging to see the field moving toward patient‑friendly options, especially for those who have struggled with the old tablets for years.
Paul Luxford
December 30, 2025 AT 09:14The safety data from the EMA review will be crucial in shaping future prescribing guidelines.
Nic Floyd
January 8, 2026 AT 15:27Yo fam, the PK/PD profile of the new ER capsule is lit 🚀-steady Cmax, reduced Tmax, and a sweet Vd that mitigates D2‑central exposure 😎.
Erik Redli
January 17, 2026 AT 21:41All this hype is just a marketing ploy; we’ll still see the same dystonia rates until long‑term neurologic trials prove otherwise.
Jacqui Bryant
January 27, 2026 AT 03:54New delivery methods could finally make metoclopramide tolerable for most patients.