When you take a pill, you trust it won’t hurt you. But drugs don’t always behave the same in every person—that’s where drug safety monitoring, the ongoing process of tracking harmful side effects and unexpected reactions to medications after they’re approved. Also known as pharmacovigilance, it’s the quiet system that catches problems clinical trials miss. Think of it like a smoke detector for medicine: it doesn’t stop fires from starting, but it alerts you before it’s too late.
Most drug side effects show up in trials with a few thousand people. But once millions start taking a drug, rare reactions appear—like a sudden drop in white blood cells, liver damage from a common painkiller, or seizures triggered by a new combination. That’s why agencies like the FDA and WHO rely on real-world reports from doctors, patients, and pharmacies. adverse drug reactions, harmful, unintended responses to medications at normal doses are logged, analyzed, and sometimes lead to black box warnings or recalls. It’s not guesswork. It’s data—from hospital records, patient apps, and even social media posts flagged by AI.
Drug safety monitoring doesn’t just track old drugs. It’s critical for generics too. Just because a generic is FDA-approved doesn’t mean everyone reacts the same. Small differences in fillers or how the pill breaks down can cause serious issues in people on narrow therapeutic index drugs like warfarin or levothyroxine. And when medications are stored in extreme heat—like in military deployments or hot warehouses—their chemistry can change. That’s why medication safety, the practice of ensuring drugs are stored, taken, and monitored correctly to prevent harm isn’t just about the pill itself. It’s about the whole chain: how it’s made, shipped, stored, prescribed, and taken.
And then there are the interactions. Two safe drugs together can become dangerous. A common antihistamine might increase fall risk in seniors. A blood pressure pill might lose its punch if taken with too much salt. These aren’t rare accidents—they’re predictable patterns, and drug interactions, when two or more medications affect each other’s action in the body, often through liver enzymes or absorption pathways are one of the top reasons people end up in the ER. Monitoring systems now track these combinations across millions of prescriptions, flagging risky pairings before they happen.
What you’ll find here isn’t theory. It’s real stories: a soldier’s insulin going bad in desert heat, an older adult falling after taking diphenhydramine, a patient’s thyroid levels crashing after switching generics, a family discovering their medicine cabinet is full of expired pills. These aren’t edge cases. They’re the reason drug safety monitoring exists—and why you need to know how to protect yourself.

Learn how clinician portals and apps help healthcare providers detect adverse drug reactions in real time. Discover which tools work best for hospitals, clinical trials, and low-resource settings-and how to use them without burnout.
Learn the daily foot inspection checklist proven to prevent diabetic foot ulcers and avoid amputation. Follow evidence-based steps for washing, inspecting, moisturizing, and choosing safe footwear every day.
Explore the latest research, new formulations, and future directions for metoclopramide, including safety updates, combination therapies, and personalized dosing.

Over half of patients with chronic conditions skip or forget their medications due to cost, confusion, complex schedules, and fear of side effects. Understanding these barriers is the first step to better health outcomes.

Curious about chlorella? Get an evidence-based look at its nutrition, real benefits, safe dosage, side effects, and how to choose a clean, quality supplement.
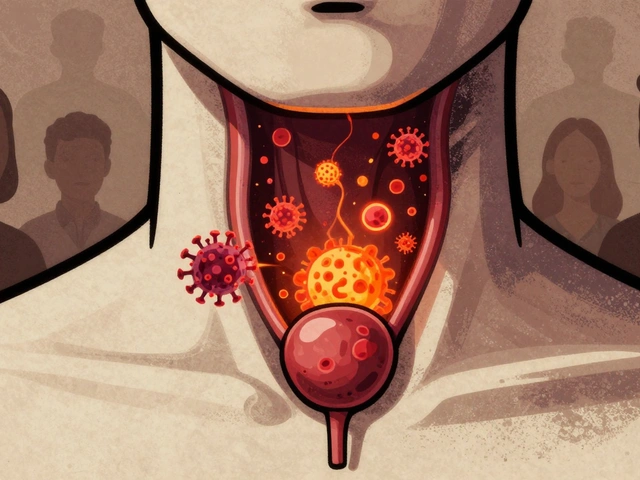
HPV causes thousands of throat and anal cancers each year in the U.S., with rising rates among men. Vaccination is the most effective way to prevent these cancers - yet coverage remains too low. Learn how HPV leads to cancer and what you can do to stop it.