When you take a pill for high blood pressure, depression, or an infection, you’re using the result of years of drug development, the process of discovering, testing, and bringing new medications to market. Also known as pharmaceutical innovation, it’s not just science—it’s a long, expensive, and often risky journey that shapes every treatment you rely on. This isn’t magic. It’s labs, clinical trials, regulatory reviews, and real people volunteering to test safety and effectiveness. And it’s changing fast—with genetics, AI, and personalized medicine now playing bigger roles than ever.
Clinical trials, structured tests that evaluate how well a drug works in humans are the make-or-break phase. Before a drug like Avanafil or Olmesartan/Amlodipine hits shelves, it goes through multiple phases: first in small groups to check safety, then in hundreds to see if it helps, and finally in thousands to confirm results. These trials don’t just measure if a drug lowers blood pressure or helps with ED—they track side effects, long-term risks, and how it compares to what’s already out there. That’s why some drugs get pulled, others get approved with warnings, and a few become go-to treatments. And now, pharmacogenomics, how your genes affect how your body responds to drugs is making trials smarter. Instead of giving everyone the same dose of Ethambutol or Cyclosporine, doctors are starting to adjust based on your DNA to avoid toxicity or boost effectiveness.
Drug development doesn’t stop at approval. It keeps going—new formulations, better delivery methods, cheaper generics, and alternatives like those comparing Neoral to Tacrolimus or Glucovance to SGLT2 inhibitors. That’s why you see so many posts here comparing medications: because knowing the differences isn’t just helpful, it’s necessary. Whether it’s a new wakefulness agent like Modvigil or a hormone therapy like Premarin, understanding how each drug was built helps you ask better questions and make smarter choices. The next time you pick up a prescription, remember: it didn’t just appear. Someone spent years making sure it was safe, effective, and worth taking.
What you’ll find below is a collection of real stories behind the pills you use—how they evolved, what went wrong, what worked, and what’s coming next. From athlete doping risks to liver disease affecting vision, these posts don’t just list facts. They show you the human side of drug development: the trials, the trade-offs, and the breakthroughs that changed lives.
Explore the latest research, new formulations, and future directions for metoclopramide, including safety updates, combination therapies, and personalized dosing.
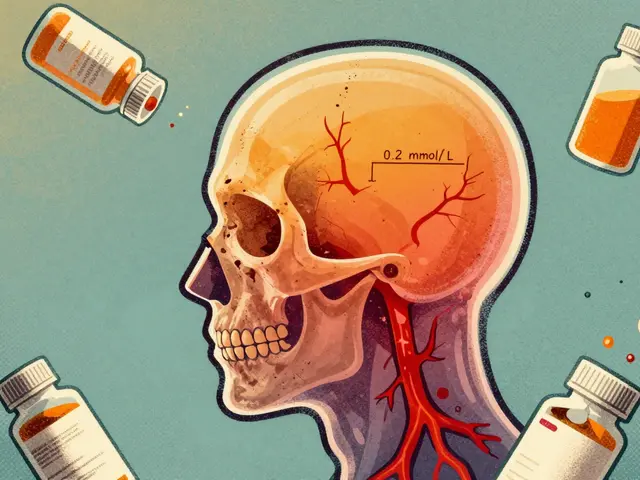
Lithium carbonate generics require careful serum level monitoring due to their narrow therapeutic index. Switching brands can alter blood levels, risking toxicity or relapse. Learn how formulation differences, dosing timing, and kidney function affect safety and effectiveness.
The FDA's Purple Book is the official guide to biosimilars and interchangeable biological drugs. Learn how it works, what the difference is between biosimilars and interchangeable products, and how pharmacists use it to make safe substitutions.
Multiple drug overdoses are deadly because substances interact unpredictably. Learn how naloxone, acetylcysteine, and emergency protocols work together to save lives when opioids, acetaminophen, or benzodiazepines are mixed.

A side‑by‑side look at Glucovance versus Metformin alone, sulfonylureas, DPP‑4, SGLT2 and GLP‑1 drugs, covering efficacy, safety, cost and when to switch.

Learn how clinician portals and apps help healthcare providers detect adverse drug reactions in real time. Discover which tools work best for hospitals, clinical trials, and low-resource settings-and how to use them without burnout.