When you see generic drug entry, a medication approved by the FDA as bioequivalent to a brand-name drug, with the same active ingredient, strength, and dosage form. Also known as generic medications, it offers the same clinical effect at a fraction of the cost — but that doesn’t mean it’s always the same for every person. The FDA requires generics to be bioequivalent, meaning they deliver the same amount of active ingredient into your bloodstream within the same time frame as the brand. But bioequivalence doesn’t guarantee identical performance in every body — especially with drugs that have a narrow therapeutic index, a small range between the effective dose and the toxic dose, where tiny changes in absorption can cause serious side effects or treatment failure.
That’s why some people report feeling different after switching from brand to generic — particularly with drugs like levothyroxine, warfarin, or phenytoin. These aren’t just minor variations. A 10% difference in absorption might be fine for ibuprofen, but for warfarin, it could mean a blood clot or dangerous bleeding. The inactive ingredients — fillers, dyes, coatings — can also affect how quickly the drug dissolves in your stomach. For someone with a rare allergy or sensitivity, even a tiny change in those ingredients can trigger a reaction. And while most people won’t notice a difference, if you’ve had stable control of your condition on one version, switching without monitoring can be risky.
It’s not just about the pill itself. drug interactions, how one medication affects the way another works in your body can change when you switch generics. Some manufacturers use different inactive ingredients that may interfere with absorption — especially when taken with food, antacids, or other meds. That’s why your doctor or pharmacist might recommend sticking to one generic brand if you’ve had good results. It’s not about brand loyalty — it’s about consistency in your body’s response.
What you’ll find below are real stories and facts about why generic drug entry isn’t always a simple swap. Some people swear by a specific brand-name version. Others switch without issue. The truth? It depends on your body, your meds, and your history. We’ve gathered posts that explain how the FDA approves these drugs, why some generics cause unexpected side effects, how to spot when a switch isn’t right for you, and what to ask your pharmacist before accepting a substitution. Whether you’re on thyroid medication, blood thinners, or seizure drugs, this collection gives you the tools to make smarter choices — not just cheaper ones.

The 180-day exclusivity rule under the Hatch-Waxman Act was meant to speed up generic drug entry-but now it often delays it. Learn how patent challenges, FDA rules, and corporate strategy keep prices high and patients waiting.

Learn how to report adverse drug reactions to the FDA's MedWatch program. Understand what counts as reportable, how to fill out the form, and why your report matters for drug safety.

Explore how common performance‑enhancing drugs affect athletes' health, the key side effects, real‑world data, and safe alternatives for optimal performance.
Kombucha contains trace alcohol that can interact dangerously with medications like metronidazole, SSRIs, and diabetes drugs. Learn what levels are safe, how to spot risky products, and what to do if you're on alcohol-sensitive meds.
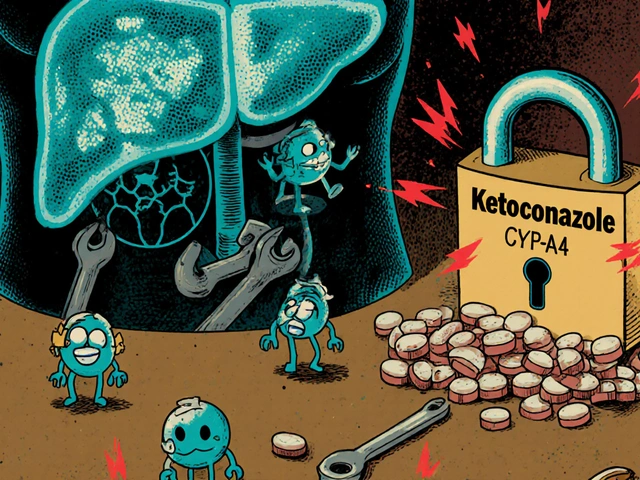
Drug-drug interactions can cause serious harm when medications clash in your body. Learn how liver enzymes, transporters, and genetics affect drug safety, and what you can do to avoid dangerous combinations.
A side‑by‑side look at Cyclogyl (cyclopentolate) versus other mydriatic eye drops, covering onset, duration, safety, cost and best‑use scenarios.