When you hear biosimilarity, the scientific standard that proves a follow-on biologic drug works like the original. Also known as biologic similarity, it's not the same as generic equivalence — and that difference can change how your treatment works. Unlike small-molecule drugs like ibuprofen or metformin, biologics are made from living cells. They’re complex, messy, and hard to copy exactly. That’s why you can’t just swap one biologic for another like you would with a generic pill. Biosimilarity is the process that proves a new version — like a biosimilar to Humira or Enbrel — behaves the same way in your body. It’s not about matching ingredients. It’s about matching outcomes: how your immune system reacts, how the drug is absorbed, how long it lasts, and whether side effects stay the same.
The FDA doesn’t approve biosimilars the same way it does generics. For generics, they just check that the active ingredient matches and the body absorbs it the same way — that’s bioequivalence, the standard for small-molecule generic drugs to prove they work like the brand-name version. For biosimilars, they run dozens of tests: protein structure, purity, stability, and even how it binds to cells. Then they test it in real people to make sure it doesn’t cause more reactions or lose effectiveness over time. This isn’t a shortcut. It’s a full scientific review. And it’s why biosimilars cost less than the original biologics — but not as cheap as your generic aspirin. You’ll see this in posts about generic drugs, medications approved as interchangeable with brand-name versions under strict FDA rules and why some people react differently to them. A generic levothyroxine might cause a swing in your thyroid levels because of tiny differences in fillers. A biosimilar to a biologic like Remicade? The risk is lower — but not zero. That’s why switching isn’t always automatic, even if the FDA says it’s interchangeable.
What you’ll find in the posts below is a clear picture of how these rules shape your access to treatment. From how patent battles delay biosimilar entry to why some patients notice changes when switching from one biologic to its copy, these stories show the real-world impact of biosimilarity. You’ll see how drug shortages push doctors toward biosimilars, how insurance formularies treat them differently than generics, and why some pharmacies still hesitate to substitute them without a doctor’s okay. This isn’t just regulatory jargon. It’s about whether your next injection works the same way, costs less, and doesn’t surprise you with new side effects. The system is built to protect you — but only if you understand how it works.
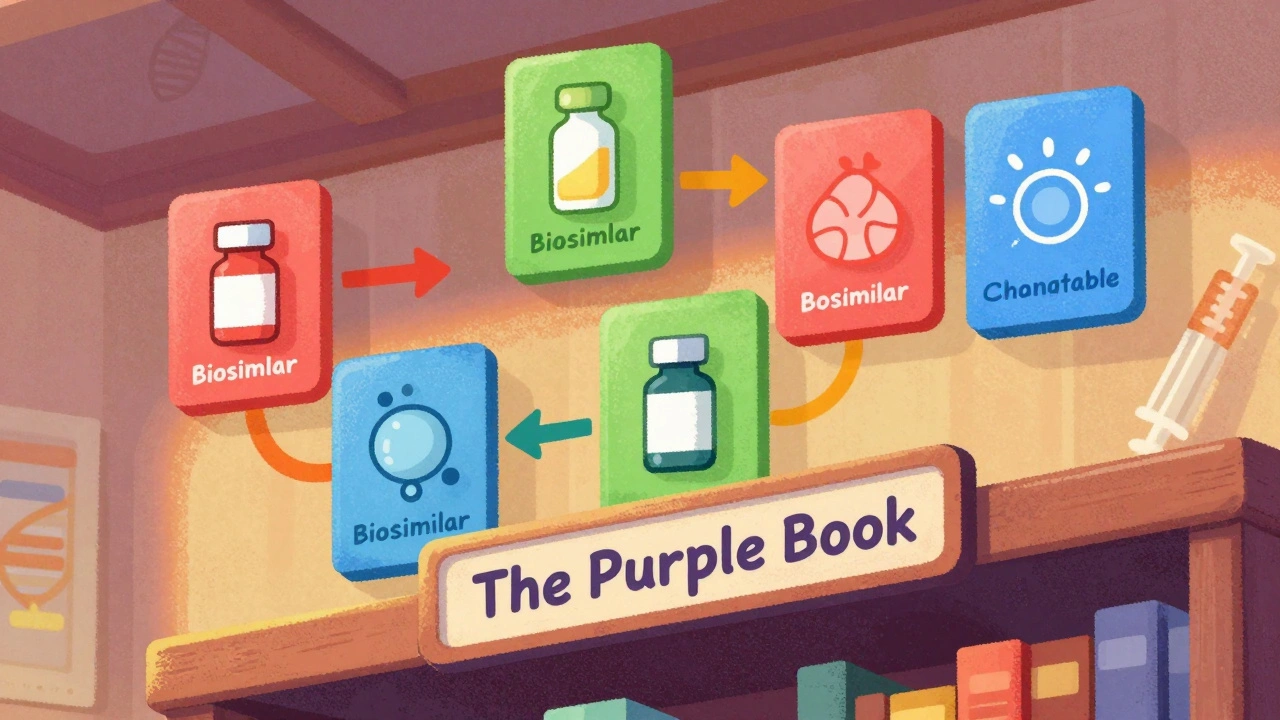
The FDA's Purple Book is the official guide to biosimilars and interchangeable biological drugs. Learn how it works, what the difference is between biosimilars and interchangeable products, and how pharmacists use it to make safe substitutions.

Learn how progesterone affects gut motility, bile flow, and microbiome health, recognize deficiency signs, and discover natural and medical ways to improve digestion.
Compare Nexium (esomeprazole) with generic alternatives like omeprazole, lansoprazole, and pantoprazole. Learn which PPI works best for acid reflux, how to switch safely, and when lifestyle changes can replace medication.
A side‑by‑side look at Cyclogyl (cyclopentolate) versus other mydriatic eye drops, covering onset, duration, safety, cost and best‑use scenarios.
Learn the daily foot inspection checklist proven to prevent diabetic foot ulcers and avoid amputation. Follow evidence-based steps for washing, inspecting, moisturizing, and choosing safe footwear every day.
When switching health plans, your generic drug coverage can save or cost you hundreds a year. Learn how formulary tiers, deductibles, and state rules impact your prescription costs-and how to avoid expensive surprises.