If you’ve ever been prescribed latanoprost for glaucoma, you might wonder how that tiny bottle got on the market. The answer starts in the 1970s when scientists were playing with prostaglandins, a family of natural chemicals that affect many body parts, including the eye. They soon realized that tweaking these molecules could make the eye’s fluid drain better, which is exactly what glaucoma patients need.
Back in the late 1970s, researchers at Pfizer noticed that a prostaglandin called PGF2α lowered eye pressure in animal studies. The problem? PGF2α caused a lot of irritation when applied to the eye. To fix that, chemists started altering its structure, trying to keep the pressure‑lowering effect while reducing side effects. After dozens of experiments, they landed on a compound that later became known as latanoprost.
In 1992, latanoprost entered human clinical trials. Volunteers with open‑angle glaucoma received eye drops once a day, and the results were striking – eye pressure dropped by about 30% on average, and patients reported only mild redness or itching. Those early successes convinced regulators that the drug was worth moving forward.
The U.S. Food and Drug Administration gave latanoprost its green light in 1996 under the brand name Xalatan. That launch was a game‑changer: for the first time, doctors had a once‑daily eye drop that was both effective and easy for patients to use. Over the next decade, the drug became the most prescribed glaucoma medication worldwide.
When the original patent expired in 2009, several generic versions hit the market. The generics kept the same active ingredient and dosage, so patients could save money without losing any benefit. Today, latanoprost is still the go‑to choice for most eye doctors treating primary open‑angle glaucoma or ocular hypertension.
Looking ahead, researchers are exploring new prostaglandin analogs that might work even faster or cause fewer side effects. But for now, latanoprost’s history shows how a simple chemistry tweak turned a lab curiosity into a daily lifeline for millions of people with glaucoma.

Explore the complete journey of latanoprost-from its chemical breakthrough and clinical trials to FDA approval and its role in modern glaucoma therapy.

Steroid myopathy causes painless, progressive muscle weakness in people on long-term corticosteroids. Learn how to recognize early signs, avoid misdiagnosis, and start safe, effective physical therapy to regain strength.

Explore how liver failure impacts vision, from jaundice and dry eye to retinal hemorrhages, and learn practical steps to protect eye health while managing liver disease.
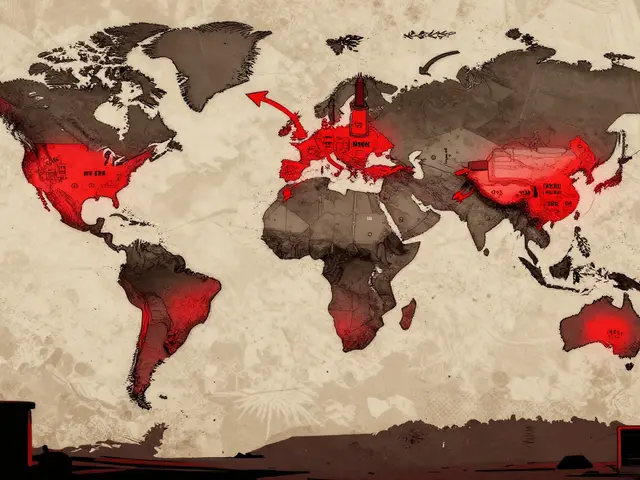
Dependence on foreign manufacturing for pharmaceutical ingredients is causing widespread drug shortages. With over 80% of active ingredients coming from just a few countries, disruptions in China or India directly impact patient access to life-saving medications.
A comprehensive side‑by‑side comparison of female Viagra (sildenafil) with Addyi, Vyleesi, generic options and herbal alternatives, covering how they work, dosing, safety and when each is best.

Learn practical ways to boost iron, B12, calcium, vitamin D and zinc absorption on vegetarian and vegan diets with food combos, cooking hacks, and gut‑health tips.